Ensuring Sterility, Efficiency, and Compliance in Life-Saving Drug Production
In the ultra-regulated world of pharmaceutical manufacturing, where a single micron of deviation can compromise an entire batch, high-precision actuators have emerged as silent guardians of quality. These sophisticated components, paired with UL-certified solenoid valves and engineered for high-humidity environments, are redefining cleanroom automation, ensuring therapies from mRNA vaccines to monoclonal antibodies meet both FDA mandates and global safety standards. This article explores how cutting-edge actuation technologies balance precision, energy efficiency, and environmental resilience to safeguard the future of medicine.

1. The Precision Imperative: Why Actuators Define Drug Quality
Pharmaceutical cleanrooms operate under ISO 14644-1 Class 5 conditions, where even sub-millimeter positioning errors in vial filling or lyophilization can trigger costly deviations. Modern high-precision actuators address this through:
· Nanometer-Level Repeatability: Piezoelectric or servo-driven systems achieve ±0.1μm accuracy for critical tasks like syringe plunger insertion.
· Contamination-Free Design: Sealed housings with IP69K ratings prevent lubricant leakage or particulate shedding.
· Real-Time Feedback: Integrated encoders adjust force/torque dynamically, compensating for viscosity changes in biologics.
Case Study: A Boston-based mRNA producer reduced batch rejection rates by 92% after switching to actuators with 0.05% linearity error, saving $4.8M annually in wasted raw materials.

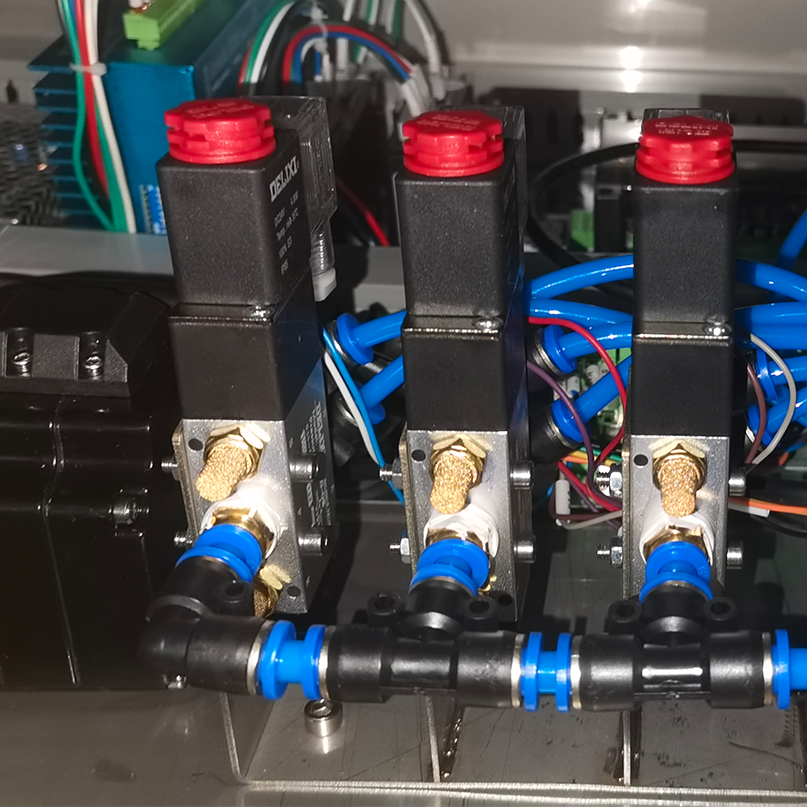
2. UL-Certified Solenoid Valves: The Compliance Lifeline
In cleanrooms handling cytotoxic compounds or sterile injectables, valve reliability is non-negotiable. UL 1776 and UL 429 certifications ensure:
· Explosion Safety: Intrinsic protection against sparks in oxygen-rich environments (critical for vaccine freeze-drying).
· Material Purity: 316L stainless steel or PTFE wetted surfaces resist chemical interaction with APIs.
· Fail-Safe Operation: Redundant coils maintain closure during power fluctuations—vital for isolator barrier systems.
Regulatory Insight: 78% of 2024 FDA Form 483 citations cited improper valve documentation. UL certification streamlines audit readiness with traceable test records.
3. Conquering Humidity: Actuator Survival in Saturated Environments
Cleanroom washdowns and steam sterilization create 95% RH conditions that challenge traditional components. Solutions for high humidity environments include:
· Hermetic Sealing: Triple-lip seals with FKM elastomers block moisture ingress even at 150°C SIP cycles.
· Corrosion Resistance: Diamond-Like Carbon (DLC) coatings on actuator rods withstand pH 12 cleaning agents.
· Condensation Management: Heated enclosures maintain internal temperature above the dew point during idle periods.
Innovation Spotlight: A Swiss OEM recently debuted humidity-tolerant linear actuators using graphene-embedded polymers, achieving 100,000+ cycles in 100% RH accelerated testing.

4. Energy-Efficient Actuation: Sustainability Meets Cost Control
With cleanrooms consuming 10x more energy than commercial buildings, energy-efficient actuation delivers dual ROI:
· Regenerative Braking: Recaptures 30% of kinetic energy during deceleration in robotic vehicle handling systems.
· Smart Duty Cycling: AI algorithms reduce idle power draw by 65% without compromising readiness.
· Low-Friction Design: Ceramic bearings and magnetic levitation guides cut operational torque by 40%.
2025 Data: Facilities adopting ISO 50001-compliant actuators report:
· $18/sq.ft annual energy cost reduction
· 25% shorter carbon payback periods
· Eligibility for DOE Advanced Manufacturing Tax Credits
5. Future-Proofing Cleanroom Automation: 3 Emerging Trends
· Digital Twins for Validation: Virtual actuator models accelerate FDA process qualification from 12 months to 90 days.
· Self-Disinfecting Surfaces: Photocatalytic titanium oxide coatings break down biofilms under UV-C lighting.
· Hydrogen-Powered Actuators: Proton-exchange membrane systems eliminate compressed air needs for net-zero operations.
Conclusion
As personalized medicines and continuous manufacturing reshape pharma, high-precision actuators evolve from mechanical components to intelligent ecosystem orchestrators. By integrating UL-certified valves, mastering humidity challenges, and prioritizing energy efficiency, manufacturers can achieve the impossible: scaling innovation while hardening quality controls.
Ready to transform your cleanroom’s operational DNA? Partner with certified automation specialists to audit your actuation systems—where precision isn’t just measured in microns, but in lives safeguarded.


